Bevarest (Bevacizumab) 100mg & 400mg Injection-uses,dose,side effects,price india
BEVAREST
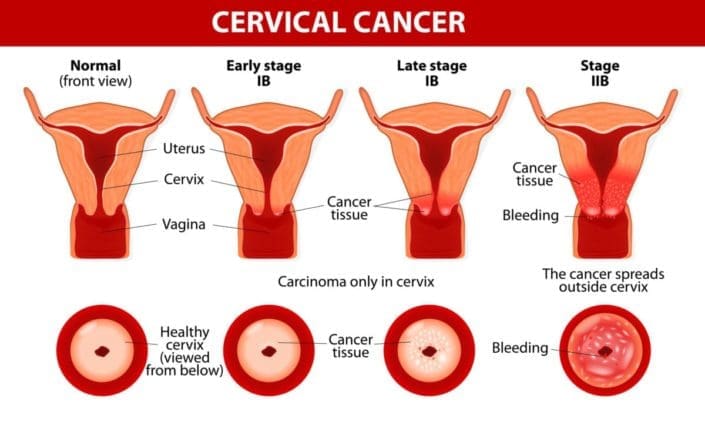 |
CERVICAL CANCER |
DESCRIPTION
Bevacizumab is a drug sold under the name of Bevarest 400mg and which is categorized as monoclonal anti-body & anti-angiogenesis
When Bevarest 400mg Injection concomitant use with human monoclonal antibody IgG1, that circumstance and prohibits the biological action of human vascular endothelial growth factor (VEGF)
Bevarest is indicated for the treatment of some of the conditions like Metastatic colon or rectal cancer Non-squamous, non-small cell lung cancer Glioblastoma Metastatic renal cell cancer Metastatic cervical cancer Epithelial ovarian, fallopian tube or peritoneal cancer
MECHANISM OF ACTION
Bevarest 400mg comprises an active compound like Bevacizumab which binds to VEGF and avoid the communication of VEGF to its receptors like Flt-1 & KDR) present on the surface of the cells This interaction prevents endothelial cell multiplication and new blood vessel production occurs Hence in counts cessation of metastatic cancer cells development happens
 |
BEVAREST 400mg |
Brand : Bevarest
Ingredients : Bevacizumab
Strength : 400mg/16ml & 100mg/4ml
Manufactured : Emcure Pharmaceuticals
Package : 400mg of Bevacizumab containing vial
Distribution:
The volume of distribution is 2.9 (22%) L
Elimination
The Bevacizumab half life period is 20days (11 to 50days)
KEY POINT
Avoid administration of Bevarest 400mg Injection before at least 28 days following surgery and the wound is completely curedMETASTATIC COLORECTAL CARCINOMA
Concomitant use with 5-fluorouracil based chemotherapy is the usual dosage of Bevarest : While concomitant use with bolus IFL the dose is 5mg/kg of Bevarest for every 2 weeks IV. While in combination with FOLFOX4 the dose is 10mg/kg ofBevarest for every 2 weeks IV 5mg/kg IV Bevarestfor every 2 weeks or 7.5 mg/kg Bevarest as IV every 3 weeks by concurrently used with fluoropyrimidine Irinotecan or fluoropyrimidine oxaliplatin based therapyNON-SMALL CELL LUNG CANCER
While combining with carboplatin and paclitaxel : The Bevarest usual dosage is 15mg/kg IV for every 3 weeks byGLIOBLASTOMA
For every 2 weeks : 10mg/kg of Bevarestadministered IVMETASTATIC CERVICAL CANCER
Combining with paclitaxel and cisplatin or with paclitaxel and topotecan : The drug Bevarest usual dosage is 15mg/kg of Bevarest given intravenously for every 3 weeks
METASTATIC RENAL CELL CANCER
Concurrent use with interferon alfa : The Bevarestusual dosage is 10mg/kg IV for every 2 weeksEPITHELIAL OVARIAN, FALLOPIAN TUBE OR PERITONEAL CANCER
Platinum opposing: Concurrent use with paclitaxel, pegylated liposomal doxorubicin or topotecan :The Bevarest regular dosage is 10mg/kg of Bevarest for every 2 weeks Or Combining with topotecan : The Bevarest regular dosage 15mg/kg of Bevarest given through IV for every 3 weeks Platinum responsive concomitant with carboplatin and paclitaxel for 6 to 8 cycles : The drug Bevarestrecommended dosage of is 15mg/kg given IV for 3 weeks Combining with gemcitabine & carboplatin for 6 to 10 cycles : The Bevarest recommended dosage is 15mg/kg of Bevarest given IV for 3 weeksPREPARATION & ADMINISTRATION
Bevarest 400mg Injection is intravenous solution At initial infusion: given IV infusion over 90 minutes Following infusions: give second infusion over 60 minutes, if tolerated Administer all following infusion over 30minutes Bevarest IV infusion is prepared in aseptic condition Bevarest 400mg containing 16ml solution whereas 100mg containing 4ml Bevarestdilute into 100ml of 0.9% NS Do not dilute with dextrose solution Dispose the remaining portion of medicine which is left in a vial
FOR FURTHER INFO
MOBILE NO : +91-9940472902EMAIL : millionhealthpharmaceuticals@gmail.com
WEBSITE URL : https://millionpharma.com/bevarest.php

Comments
Post a Comment